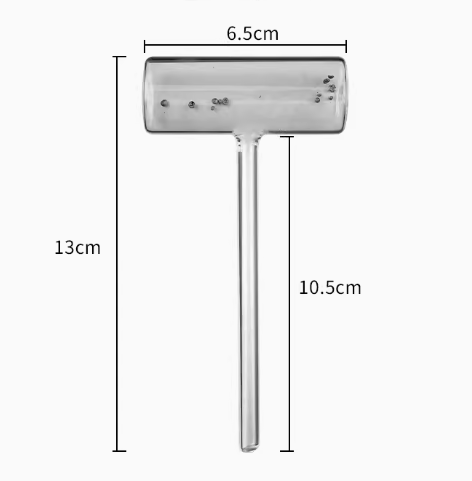
Iodine sublimation and condensation hammer tube
碘昇華管
HK$20.00
Iodine sublimation and condensation are processes related to the phase change of iodine from solid to gas (sublimation) and from gas to solid (condensation). Sublimation is the direct transition of a substance from the solid phase to the gas phase without passing through the liquid phase. In the case of iodine, solid iodine crystals can sublimate when heated, resulting in the formation of iodine vapor. This process occurs because the temperature reaches the substance's sublimation point, which for iodine is around 113.7 degrees Celsius (236.7 degrees Fahrenheit).
A common setup for iodine sublimation involves using a sublimation tube. This tube is typically made of glass and has a closed end and an open end. The solid iodine is placed at the closed end of the tube, and heat is applied. As the iodine sublimes, it forms a purple vapor that fills the tube. The vapor can then be collected and used for various purposes.
Condensation, on the other hand, is the reverse process of sublimation. It involves the transition of a substance from the gas phase to the solid phase. In the case of iodine, condensation occurs when the iodine vapor cools down and reverts to solid iodine crystals. This process can be achieved by lowering the temperature or by allowing the vapor to come into contact with a cold surface.
碘昇華和冷凝是與碘從固體到氣體(昇華)和從氣體到固體(冷凝)的相變有關的過程。昇華是物質從固相直接轉變為氣相而不經過液相。 就碘而言,固體碘晶體在加熱時會昇華,形成碘蒸氣。 發生此過程是因為溫度達到了物質的昇華點,對於碘來說,昇華點約為 113.7 攝氏度(236.7 華氏度)。
碘昇華的常見設定涉及使用昇華管。 該管通常由玻璃製成並具有封閉端和開口端。 將固體碘置於管的封閉端並加熱。 當碘昇華時,它會形成充滿管子的紫色蒸氣。 然後可以收集蒸氣並用於各種目的。
另一方面,冷凝是昇華的逆過程。 它涉及物質從氣相到固相的轉變。 就碘而言,當碘蒸氣冷卻並恢復為固體碘晶體時會發生冷凝。 該過程可以透過降低溫度或讓蒸氣與冷表面接觸來實現。
Youtube short
https://www.youtube.com/shorts/7lo5cdRRdrk