Sodium bicarbonate
碳酸氫鈉
HK$120.80
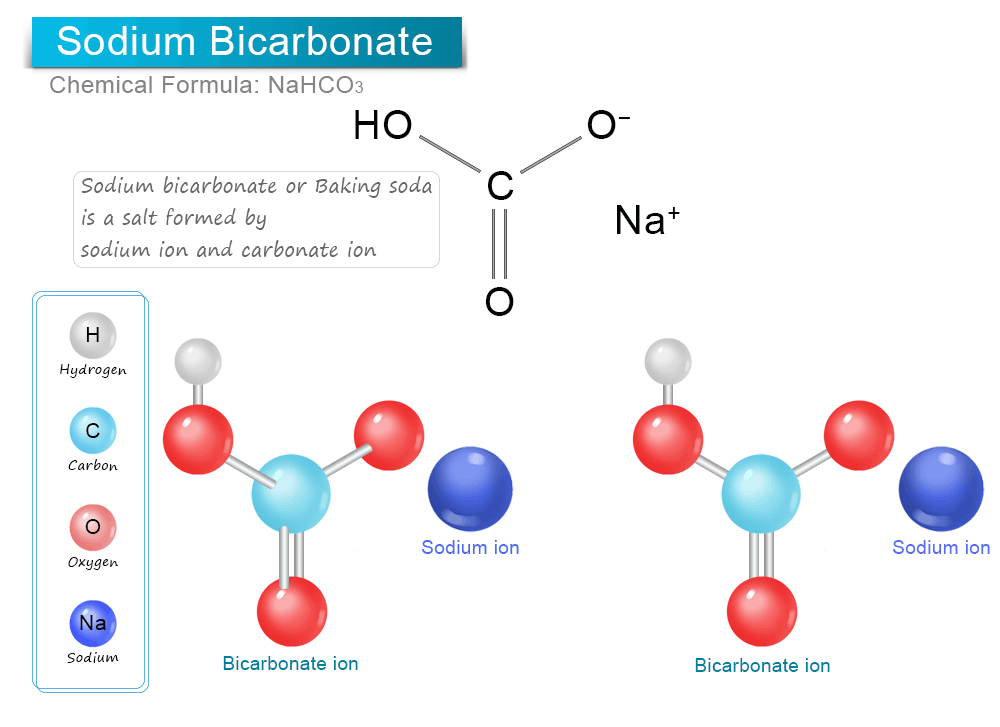
NaHCO3, >99.5%, CAS : 144-55-8
Sodium bicarbonate, commonly known as baking soda, has several applications in secondary school science experiments. Here are a few examples:
1. Chemical reactions: Sodium bicarbonate can be used to study various chemical reactions. For example, students can mix sodium bicarbonate with vinegar to observe the production of carbon dioxide gas, which causes bubbling. This experiment demonstrates the reaction between an acid (vinegar) and a base (sodium bicarbonate).
2. pH testing: Sodium bicarbonate can be used as a pH indicator. Students can dissolve sodium bicarbonate in water and use it to test the acidity or alkalinity of solutions. When added to an acidic solution, sodium bicarbonate will cause effervescence or bubbling due to the release of carbon dioxide gas.
3. Fire extinguishing: Sodium bicarbonate can be used to illustrate the principle of using it as a fire extinguisher. Students can ignite a small amount of flammable material and then carefully sprinkle sodium bicarbonate on the flames to observe how it suppresses the fire by releasing carbon dioxide gas and smothering the flames.
4. Volcano eruption: Sodium bicarbonate can be used to create a volcanic eruption model. By combining sodium bicarbonate, vinegar, and a touch of food coloring in a model volcano, students can observe the resulting chemical reaction, which produces carbon dioxide gas and causes the volcano to "erupt."
These experiments provide students with hands-on experience in chemical reactions, pH testing, fire safety, and volcanic eruptions, helping them understand key concepts in chemistry and develop practical laboratory skills.
Sodium bicarbonate碳酸氫鈉,俗稱小蘇打,在中學科學實驗中有許多應用。 這裡有一些例子:
1.化學反應:碳酸氫鈉可用於研究各種化學反應。 例如,學生可以將碳氫鈉與醋混合,觀察二氧化碳氣體的產生,二氧化碳氣體會造成氣泡。 此實驗展示了酸(醋)和鹼(碳酸氫鈉)之間的反應。
2.pH測試:碳酸氫鈉可用作pH指示劑。 學生可以將碳氫化合物氫鈉溶解在水中,並用它來測試溶液的酸性或鹼度。 當添加到酸性溶液中時,碳酸氫鈉會因釋放二氧化碳氣體而引起泡騰或冒泡。
3.滅火:可用碳酸氫鈉來說明其用作滅火劑的原理。 學生可以點燃少量易燃材料,然後小心地將碳酸氫鈉撒在火焰上,觀察它如何透過釋放二氧化碳氣體並窒息火焰來抑制火勢。
4.火山爆發:碳酸氫鈉可用於創建火山爆發模型。 透過將碳
酸氫鈉、醋和少量食用色素混合在火山模型中,學生可以觀察由此產生的化學反應,該反應會產生二氧化碳氣體並導致火山「噴發」。
這些實驗為學生提供化學反應、pH 測試、消防安全和火山爆發方面的實務經驗,幫助他們理解化學的關鍵概念並培養實用的實驗室技能。
Chemical Structure made available to public from PubChem
Nomenclature according to IUPCA standard
All prices are in Hong Kong Dollars (HKD)
Refer HERE for Purity representation