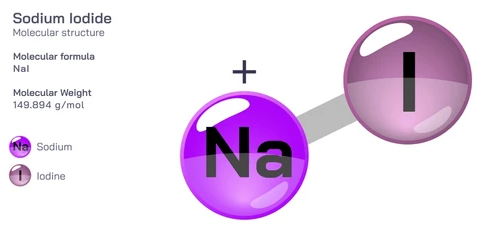
Sodium iodide
碘化鈉
HK$533.00
NaI, AR 99.5% , CAS : 7681-82-5
Sodium iodide (NaI) is an inorganic compound consisting of sodium (Na+) ions and iodide (I-) ions. It is a white, crystalline solid that is highly soluble in water.
From a chemical perspective, sodium iodide is widely used as a source of iodide ions in chemical reactions. The iodide ion exhibits various reactivities and can participate in redox reactions, complexation reactions, and organic synthesis.
Sodium iodide finds extensive applications in the medical field. It is commonly used as a supplement to prevent iodine deficiency, which can lead to thyroid disorders. It is also used in the treatment of certain thyroid conditions and as a radiographic contrast agent in medical imaging.
In addition, sodium iodide is employed as a scintillation material in radiation detectors. It can emit light when exposed to ionizing radiation, allowing for the detection and measurement of radioactive substances.
From a safety perspective, sodium iodide should be handled with care, as it can be toxic in high doses and may cause skin and eye irritation.
Sodium iodide (NaI) 碘化鈉 是一種由鈉 (Na+) 離子和碘 (I-) 離子組成的無機化合物。 它是一種白色結晶固體,極易溶於水。
從化學角度來看,碘化鈉廣泛用作化學反應中的碘離子源。 碘離子表現出多種反應活性,可以參與氧化還原反應、絡合反應和有機合成。
碘化鈉在醫學領域有廣泛的應用。 它通常用作預防碘缺乏的補充劑,碘缺乏會導致甲狀腺疾病。 它也用於治療某些甲狀腺疾病,並用作醫學成像中的放射線造影劑。
此外,碘化鈉也用作輻射探測器中的閃爍材料。 當暴露於電離輻射時它可以發光,從而可以檢測和測量放射性物質。
從安全角度來看,碘化鈉應小心處理,因為高劑量時可能有毒,並可能引起皮膚和眼睛刺激。
Chemical Structure made available to public from PubChem
Nomenclature according to IUPCA standard
All prices are in Hong Kong Dollars (HKD)
Refer HERE for Purity representation